CORROSION
Corrosion is a natural phenomenon and is the reaction of metals or metal alloys with water and air in the environment. Corrosion results in the conversion of metals to metal oxides, such as iron metal to orange-red iron oxide or "rust". Corrosion is undesirable when it happens to metals in equipment supporting or making convenient our daily life, e.g., vehicles, bridges, drinking water distribution systems, etc. A huge amount of money is spent annually for the replacement or maintenance of public and private infrastructure due to corrosion1.
Corrosion of heat exchanger metal or metal alloy components is a big concern in thermoelectric power plant cooling water systems. It can cause the decrease of heat exchange efficiency by forming corrosion products on the metal or metal alloy surfaces, or cause penetration of heat exchanger tubing thus contaminating high purity boiler water with low quality cooling water.
Corrosion of cooling system piping is less of a concern than that of heat exchanger components. However, the piping materials still need to be protected since the amount piping materials is relatively massive compared to that for heat exchanger units. Commonly used metal and metal alloy materials in cooling water systems are shown in Table 1.
TABLE 1. Â Common alloys used in cooling water systems |
|
Alloy |
Common uses |
Carbon steel, low-alloy steels |
Transfer lines, heat exchanger shells, baffles, pump components, heat exchanger tubing, fan blades and shrouds, valves, screens, fasteners |
Cast iron |
Pump housings and impellers, valves, plumbing fixtures, large diameter pipe |
Galvanized steel |
Cooling tower components, fan blades and shrouds, transfer pipes, plumbing fixtures |
Bronzes |
Bearings, pump impellers, special-purpose tubing, heat exchanger tubing, screens |
Copper and brasses |
Heat exchanger tubing, bearings, valve components, gaskets, brewing equipment |
Cupronickel |
Heat exchanger tubing |
Stainless steels |
|
   300 series |
Plate-and-frame exchangers, heat exchanger tubing, pumping impellers and housings, heat exchanger shells, brewing equipment, drying equipment, oil refinery still tubes, pulp and paper equipment |
   Duplex |
Stress-corrosion-cracking-resistant tubing |
   400 series |
Special-purpose heat exchanger tubing, plate-and frame heat exchangers. |
Aluminum |
Heat exchanger tubing, transfer piping |
Molybdenum |
Furnace electrodes used to melt siliceous compounds |
Nickel |
Special-purpose heat exchangers, caustic handling |
Titanium |
Fresh and sea water condenser tubing, severe corrosion condition equipment |
Source: Herro, H.M.; Port, R.D. (1993) Nalco Guide to Cooling Water System Failure Analysis; McGraw-Hill, Inc.: New York. |
|
Â
Corrosion of metal alloys in contact with water is an electrochemical process. Anode and cathode areas form on the metal surface due to the difference of electrical potentials on the surface of an alloy. The area having the lower electrical potential is the anode and the area with the higher potential is the cathode. Corrosion involves oxidation in which electrons are released at the anode. The cathode receives electrons from an external circuit and reduction occurs. When equilibrium is reached the anodic and cathodic potentials attain the same value, called the corrosion potential, the current passing is called the corrosion current. Aqueous solution serves as the electrolyte to complete the internal circuit. Figure 1 shows a schematic of mild steel corrosion in contact with recirculating cooling water.
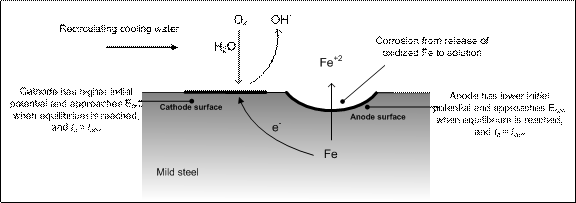
FIGURE 1.  Schematic of mild steel corrosion in contact with recirculating cooling water. On the metal surface, cathode and anode surface form due to the difference of electrical potentials. At equilibrium, reductive current (Ia) is equal to oxidative current (Ic) and is defined as corrosion current (Icorr). (Source: Hsieh, 2009)
Corrosion of metals and metal alloys in cooling systems is influenced by many operational conditions, such as cooling water temperature, cooling water flow velocity, and especially cooing water quality. The most important water quality parameters influencing corrosion inhibitor concentrations (e.g., phosphate), aggressive chemical species (e.g., chlorine, ammonia), and microbes, as illustrated in Figure 2.   Â
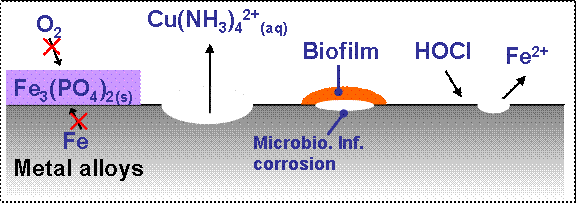
FIGURE 2. Â A schematic showing the water quality parameters that can influence corrosion of metals or metal alloys in cooling water systems. (Source: Hsieh, 2009)
Corrosion control in cooling water systems is generally practiced by employing corrosion inhibitors, which generally form a very thin protective layer on the metal and metal alloy surfaces.
Most knowledge of corrosion control of thermoelectric power plant cooling water systems is built upon the experience of using freshwater as makeup water. When using treated municipal wastewater or other ion-quality as cooling system makeup comes into practice due to freshwater shortage, the corrosion control strategies will be different than our previous experience. The understanding of corrosion behaviors of metals and metal alloys in the context of using ion quality waters as cooling makeup is really important in order to apply effective corrosion control strategies.Â
Footnotes:
1. It has been estimated that the annual direct cost of corrosion among industrial sectors in the U.S. exceeds $100 billions. (Koch, G,H.; Brongers, M.P.H.; Thompson, N.G.; Virmani, Y.P.; Payer, J.H. (2001) Corrosion Costs and Preventive Strategies in the United States. FHWA-RD-01-156. National Technical Information Service: Springfield, VA.)