SCALING
Scaling refers to the deposition of crystalline mineral salts onto a hard surface. The scale layers are usually hard and adherent. Scaling is a common issue in drinking water distribution systems and cooling water systems. Scales can serve as sites for adsorption and coprecipitation of other chemicals and thus accelerate the formation of fouling.Â
Scale formation on the surface of thermal conductive metal or metal alloy surfaces of heat exchangers of cooling water system is undesirable. Scales reduce the rate of heat transfer and increase the hydraulic head loss, both negatively impact the electricity generation efficiency of a power plant.
The scale formation is a process of several steps: mineral ions clustering, nucleation, crystal growth, flocculation, and deposition. Generally, a status of supersaturation of a mineral salt is necessary for it to precipitate. Supersaturation means the concentration of the mineral salt exceed its solubility in a particular aqueous solution. Figure 1 illustrates the process of mineral precipitation and deposition.

FIGURE 1. The process of scale formation of a mineral salt in aqueous environment.
The most common scales of interest are calcium carbonate (calcite) and calcium phosphate:
                       Ca(+2) + CO3(-2) = CaCO3(s)
                       3Ca(+2) + 2PO4(-3) = Ca3(PO4)2(s)
Both CaCO3(s) and Ca3(PO4)2(s), have greater tendency to precipitate at higher temperature and thus heat exchanger metal or metal alloy tubing surfaces provide an excellent environment for scales to deposit and grow.Â
Generally, scale formation is pH dependent. The higher the pH, the greater the tendency for scale formation. Both CaCO3(s) and Ca3(PO4)2 show this characteristic. Thus, pH adjustment is a common approach for controlling scale formation.Â
The other very common approach to control scaling is through addition of scaling inhibitors. A scaling inhibitor can function in one or more than one of the following ways: sequestration, crystal distortion, dispersion, and surface conditioning. The four aspects are illustrated in Figure 2.
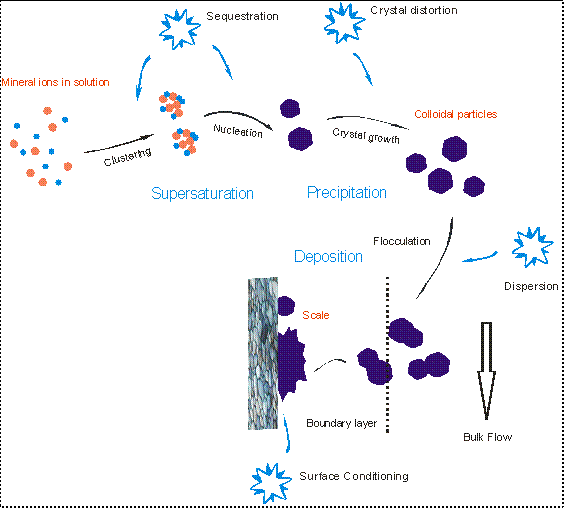
FIGURE 2. The functioning of scaling inhibitors: sequestration, crystal distortion, dispersion, and surface conditioning.
When using treated municipal wastewater as power plant cooling system makeup water, scaling is much more challenging because of the high concentrations of calcium, carbonate species, and phosphate in the wastewater. Thus, physical/chemical pretreatments of the wastewater effluent before entering into cooling systems or higher dosage of scaling inhibitors will be necessary for the scaling control.