Research Reveals Underlying Mechanism that Renders Certain High-Risk Cancers Immortal
Carnegie Mellon researchers identify droplets full of DNA repair proteins in ALT cancer cells
Media Inquiries
Researchers at Carnegie Mellon University have uncovered a key mechanism that promotes telomere elongation, a discovery that has implications for understanding and potentially treating some of the world’s deadliest cancers.
The study, published in Genes and Development(opens in new window), suggests that an alternative process that some cancer cells use to lengthen telomeres, resulting in cell immortality, involves the formation of tiny droplets full of DNA repair proteins.
Telomeres are the protective caps at the end of chromosomes, like the plastic piece at the end of a shoelace. Telomeres shorten with every cell division, until they disappear and the cell dies. Most cancer cells circumvent this process using the enzyme telomerase to continuously lengthen their telomeres, keeping the cell alive.
But some cancer cells — an estimated 10 to 15 percent — use a process called alternative lengthening of telomeres (ALT). These ALT cancers include some of the deadliest kinds, including glioblastoma, osteosarcoma, and pancreatic neuroendocrine tumors.
Because the ALT process protects telomeres in tumor cells, it is an attractive target for cancer treatments.
The Carnegie Mellon researchers, led by Huaiying Zhang(opens in new window), Eberly Family Assistant Professor of Biological Sciences, focused on a specific ALT component called SUMO.
“Using SUMO inhibitors, we were able to efficiently abolish ALT and impair the growth of these cancer cells, thus providing a possible approach for ALT cancer therapy,” Zhang said.
SUMOs (small ubiquitin-like modifiers) attach to other proteins to modify their function, from adjusting how they fold to directing where they move around the cell. They mediate numerous processes in healthy cells. In ALT cancer cells, SUMO tags DNA repair proteins, steering them to the telomeres that need to be lengthened.
Rongwei Zhao, a graduate student in Zhang’s group, used a novel approach to study how SUMO-tagged proteins behave once they are gathered at a telomere. Together with their collaborators at the University of Pennsylvania, Zhang and Zhao developed a chemical dimerization system that allows researchers to target specific proteins to specific places on a chromosome. In Zhao’s case, she dispatched SUMO to telomeres.
“We artificially enriched tons of SUMO on telomeres by adding the dimerizer, a chemical compound that brings proteins into close proximity so that they can interact,” Zhao explained.
This method enabled Zhao to continuously monitor what was happening at the telomeres in real time.
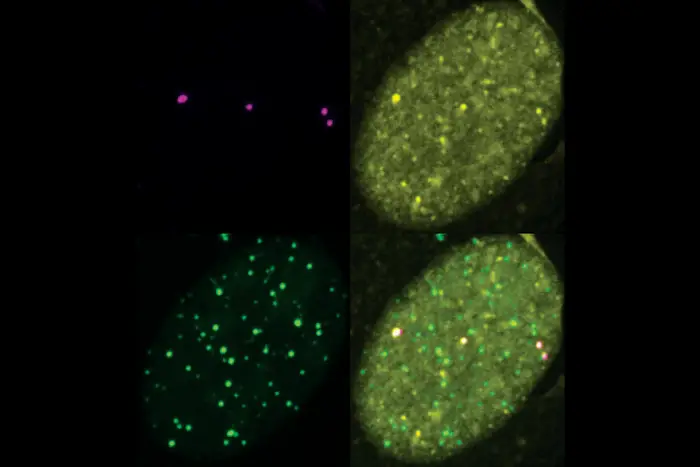
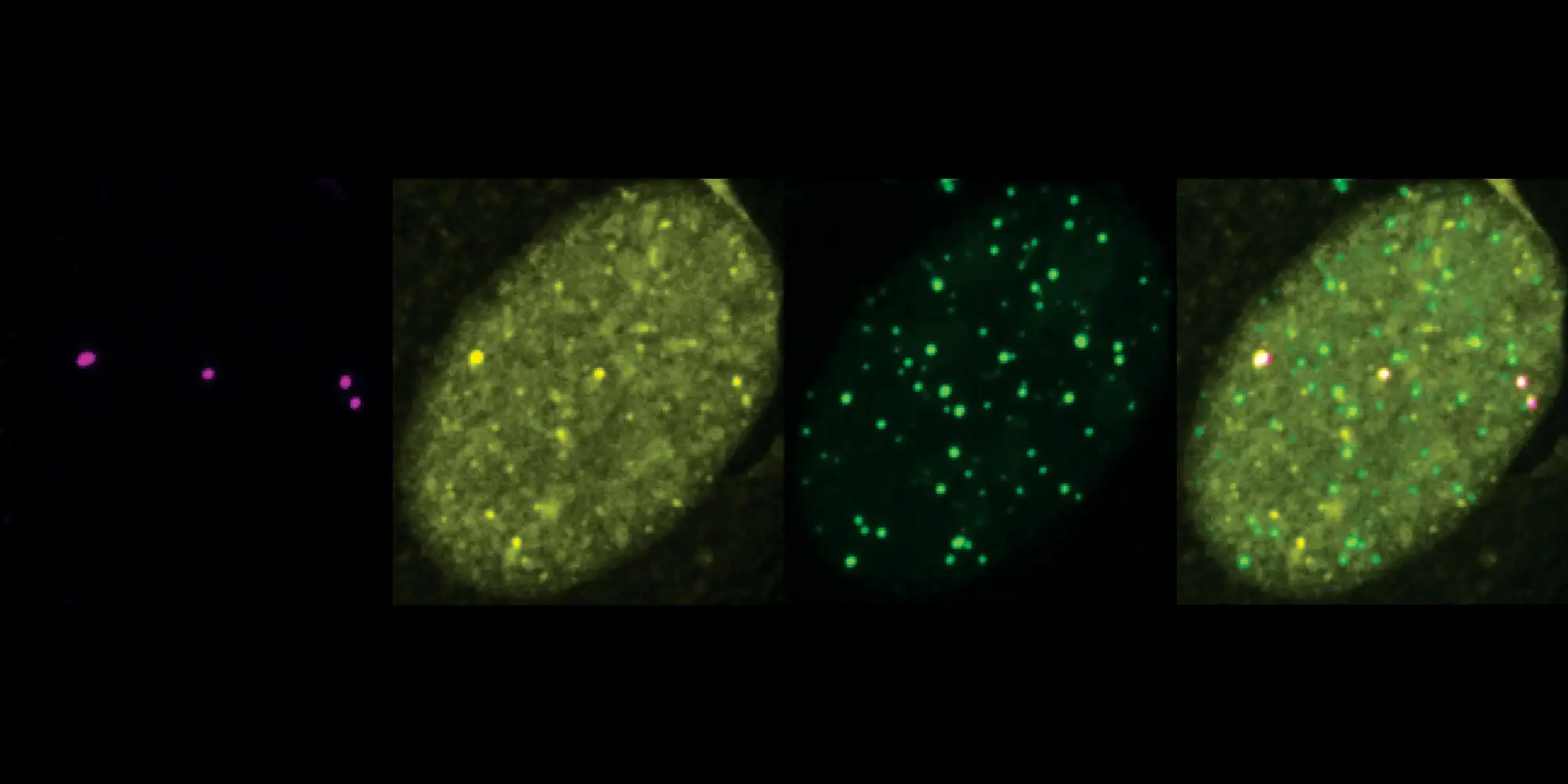
Once this conglomeration reached a critical mass, Zhao observed that the proteins separate from their cellular surroundings and clump together — similarly to how oil separates from water. The process, called phase separation, plays an important role in many biological processes. In this instance, the SUMO-rich droplets recruit DNA repair factors, which then bump into each other, fuse and provide templates to work together to elongate the telomeres.
“We induced phase separation on demand,” Zhao said. “And when we inhibited SUMO with the inhibitor, there was no phase separation and there was no recruitment of DNA repair factors.”
Their findings suggest that SUMO is the key driver for phase separation and recruitment of DNA repair factors on telomeres. It is a step toward putting the pieces together to understand how SUMOylation functions in ALT cancer cells and in other cellular processes.
According to the researchers, it may even work in a similar way in other parts of the chromosome. “If SUMOs are enriched on other genome loci, such as centromeres, it also can potentially be key to promoting other DNA factors that are involved in diverse chromosomal functions,” Zhao said.
In earlier work, Zhang, Zhao and colleagues found that SUMOylation can drive phase separation, forming a droplet on the telomere that then promotes the formation of APB (ALT-associated PML bodies). APB is known to regulate telomere elongation. For that research, they developed and then used their protein-localization tool to discover that the merging of APBs causes telomeres to cluster together.
In the current work, they ran their experiments in cells lacking PML to uncover whether SUMO still played a role in the absence of PML. Their results show that SUMO is both required and sufficient to induce ALT processes without PML, and that SUMO promotes DNA repair factor enrichment at telomeres independent of APBs.
In addition to Zhao and Zhang, co-authors include: Meng Xu, associate research fellow, and Xiaoyang Yu, postdoctoral fellow, from Carnegie Mellon; Anne R. Wondisford and Roderick J. O’Sullivan from the University of Pittsburgh Medical Center Hillman Cancer Center, University of Pittsburgh; Rachel M. Lackner and David M. Chenoweth from the University of Pennsylvania; Jayme Salsman and Graham Dellaire from Dalhousie University; and Xiaolan Zhao from the Memorial Sloan Kettering Cancer Center.
This work was supported by National Institutes of Health (NIH) grants (U01CA260851 to H.Z., GM118510 to D.M.C., and R35 GM145260 to X.Z.) and a Project Grant from the Canadian Institutes of Health Research (CIHR) (PJT-156017 to G.D.). A.R.W. receives support from NIH training award T32 GM133332 (Department Pharmacology and Chemical Biology, University of Pittsburgh) and Ruth L. Kirschstein National Research Scientist Training Award CA278287.