Disease progression in Pulmonary Arterial Hypertension
Pulmonary arterial hypertension invovles remodeling of the entire pulmonary arterial tree with narrowing of distal vessels driving up afterload on the right ventricle, leading to death or transplant. Our group uses computational tools to understand the mechanisms underlying the evolving pulmonary vasculature. These tools simulate the turnover of constituents in the vessel wall due to mechanobiological and immunological cues. For example, increased wall shear stress, which is the frictional force of blood moving across the lumen of the vessel, can initially lead to adaptive changes in radius to attempt to return the experienced force towards the normal level. But maladaptive remodeling can develop with prolonged exposure, driving a positive feedback loop that further narrows vessels.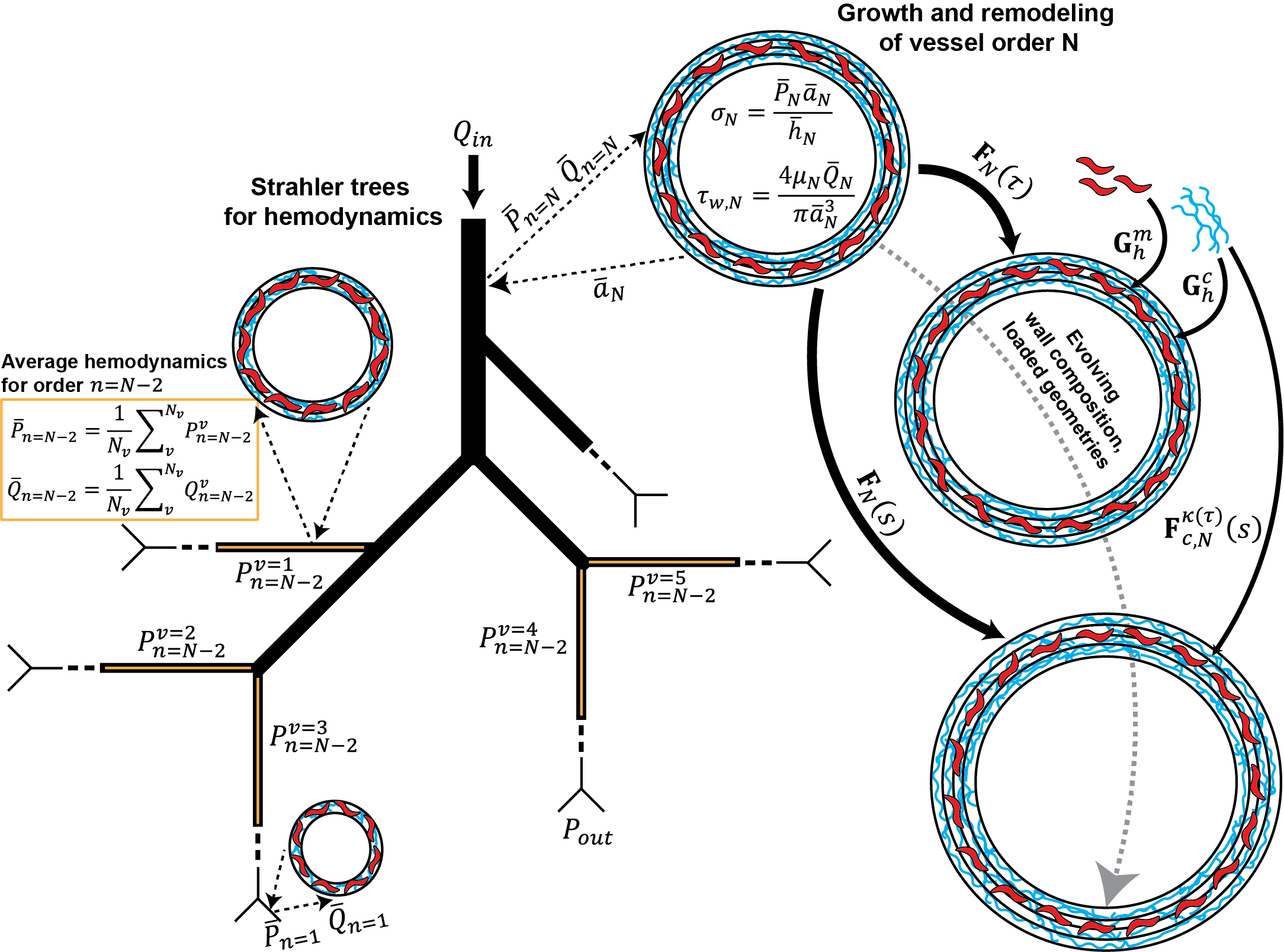
To inform our computational frameworks, we use clinical and pre-clinical data, including hemodynamic measurements from catheterization, material composition from histology and immunostaining, and geometry or morphometry from volumetric imaging. We also aim to develop novel imaging and loading techniques to undestand changes in biomechanical behavior across vessel sizes.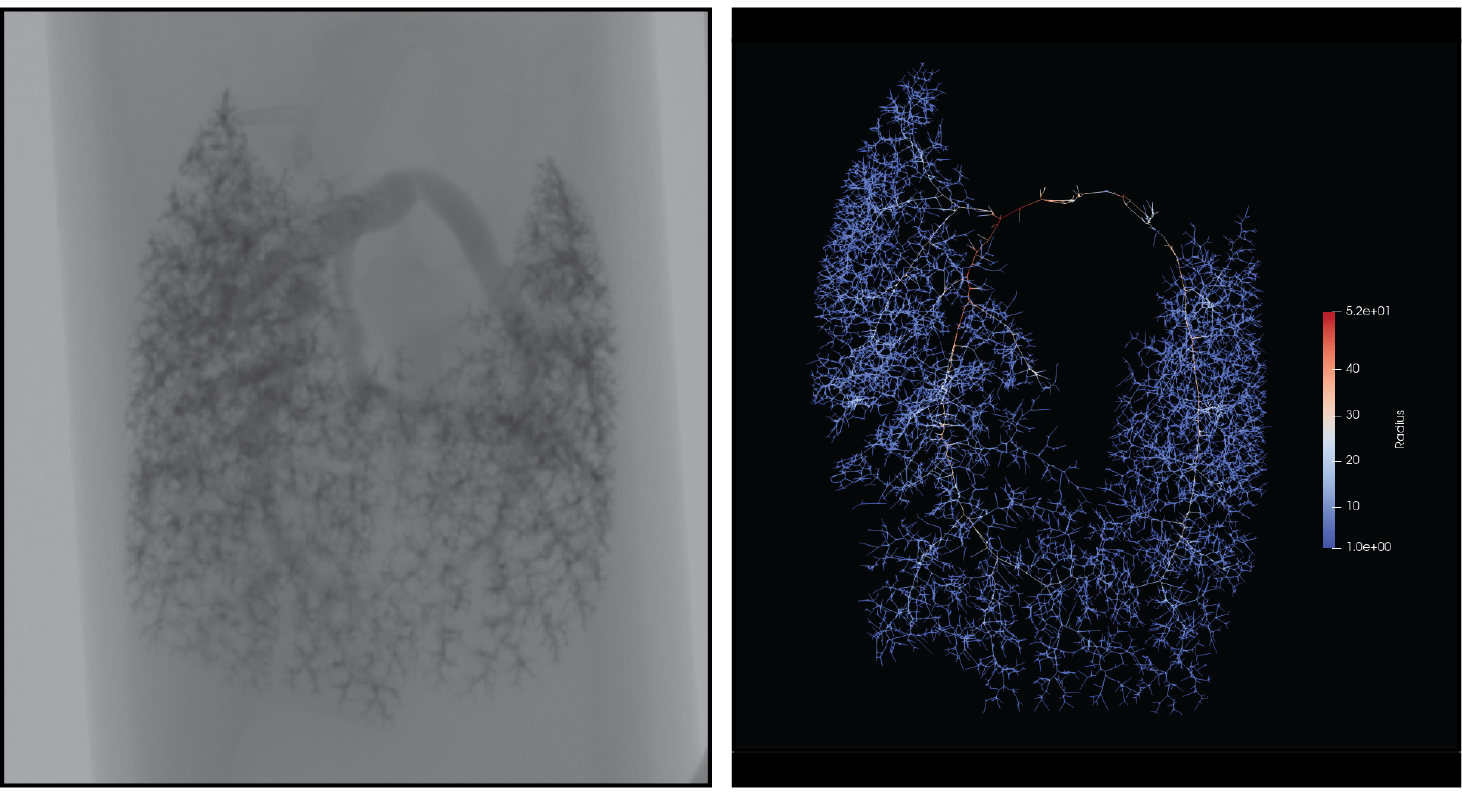
Predicting outcomes of clinical interventions in the pulmonary vasculature
Progressive pulmonary diseases often require surgery or intervention. Advanced respiratory disease or pulmonary hypertension can lead to the need for a lung transplant, where donor lungs are implanted within a recipient to restore function. We are using computational fluid dynamics to simulate varying approaches to the transplantation procedure, such as predicting the resulting pulmonary pressures after either a single or double lung transplant. Our goal is to identify donor and recipient matching criteria that are based on improved biomechanical and mechanobiological matching. In addition to lung transplantation, we are also interested in modeling other interventions, such as angioplasty to restore flow in narrowed pulmonary vessels.
Design and optimization of engineered blood vessels
For many cardiovascular diseases, there is a need for replacement blood vessels to bypass or replace those that have become likely to fail. Additionally, pediatric patients require novel grafts that will grow and change with the patient. We work to use our in silico tools to design engineered vessels that can replace or augment autologous vessels. Parameters related to fabrication are included in the simulations and can be tuned to alter the long term remodeling of a particular design. Including formal methods of optimization then also allows for identifying promising parameter sets that limit catastrophic failure and mimic normal function.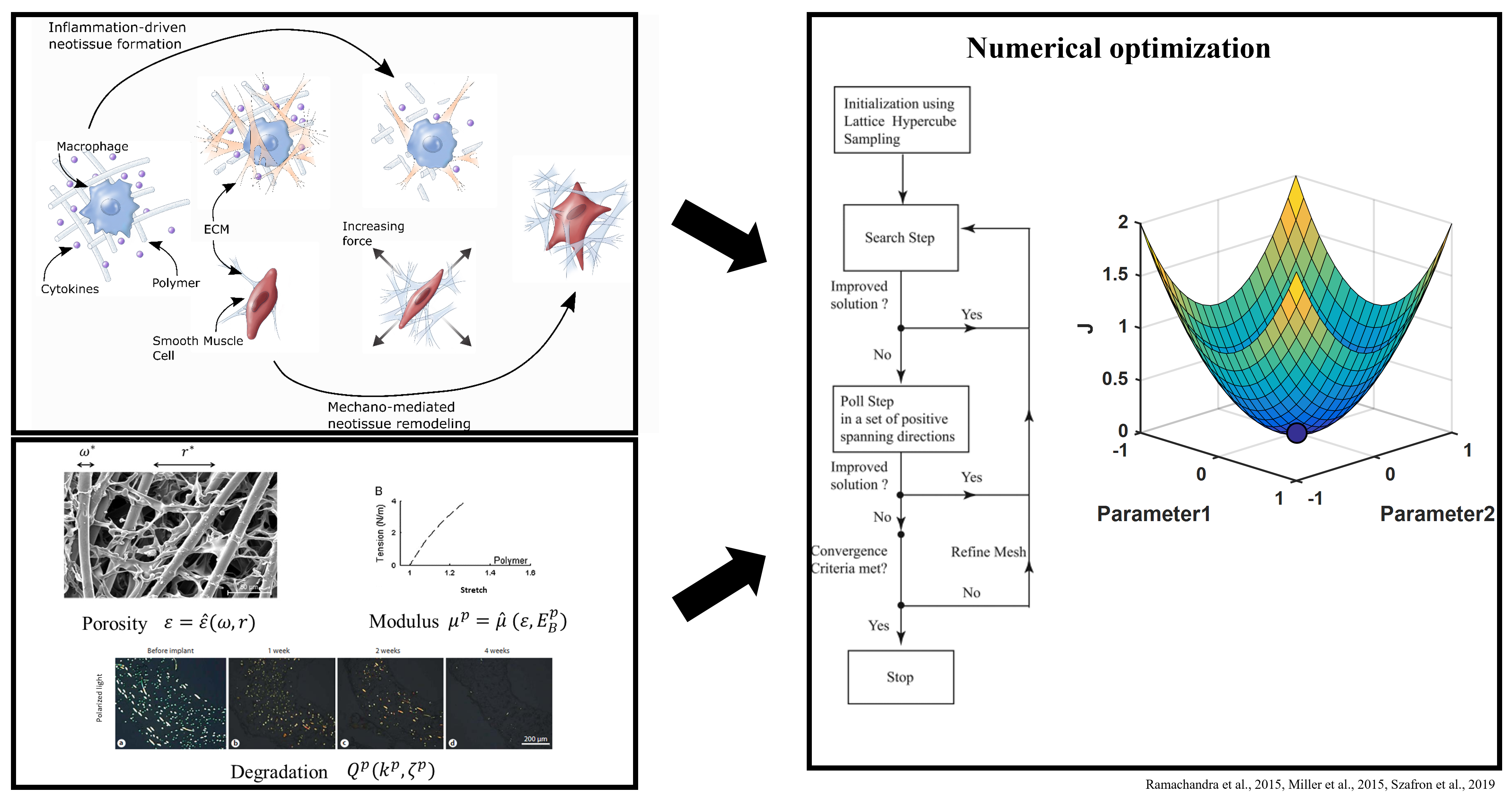 These methods use a growth and remodeling framework that simulates the graded load transfer between an implanted scaffold and infiltrating neotissue with the intent of modeling in situ vessel formation. However, there is a need to extend this approach to consider the evolution of engineered vessels in vitro, particularly those made from biopolymers using novel soft fabrication techniques. Such an approach will allow for improved design of in vitro test beds for drug development and generation of large scale tissues for implantation.
These methods use a growth and remodeling framework that simulates the graded load transfer between an implanted scaffold and infiltrating neotissue with the intent of modeling in situ vessel formation. However, there is a need to extend this approach to consider the evolution of engineered vessels in vitro, particularly those made from biopolymers using novel soft fabrication techniques. Such an approach will allow for improved design of in vitro test beds for drug development and generation of large scale tissues for implantation.
