Lung Stem Cells and Directed Morphogenesis. We are interested in understanding and engineering fundamental cellular interactions during the biofabrication of lung tissues at micrometer to centimeter scale. Native organogenesis requires orchestrated interactions between cells of the same and different lineage origins. We investigate how such interactions shape lung epithelial maturation and morphogenesis. We established the first in vitro model for human heart and lung co-development using pluripotent stem cell differentiation (Ng WH et al., 2021, Ng WH et al., 2023). We also developed airway organoid models with tunable epithelial polarity to delineate rules governing epithelial polarization and stem cell maintenance during morphogenesis, and to enable respiratory interactions with airborne pathogens and pollutants in a physiologically relevant manner (Wijesekara P et al., 2022; Wijesekara P et al., 2023). Ongoing research focuses on (1) developing lung assembloid (multi-lineage organoid assembly) incorporating epithelial, stromal, immune cells, and extracellular matrix to fully recapitulate the native lung tissue architecture along the apical-basal axis; and (2) developing additive manufacturing strategies for spatially directing lung tissue morphogenesis from stem cells.

Autonomous segregation between the cardiac and pulmonary lineages within a dual-lineage assembloid derived from pluripotent stem cell differentiation. (Ng WH, et al. 2021)

The exterior-facing motile cilia power spinning motility of the Apical-out Airway Organoid (AoAO), allowing expedited assessment of cilia pathophysiology. (Wijesekara P et al., 2022)
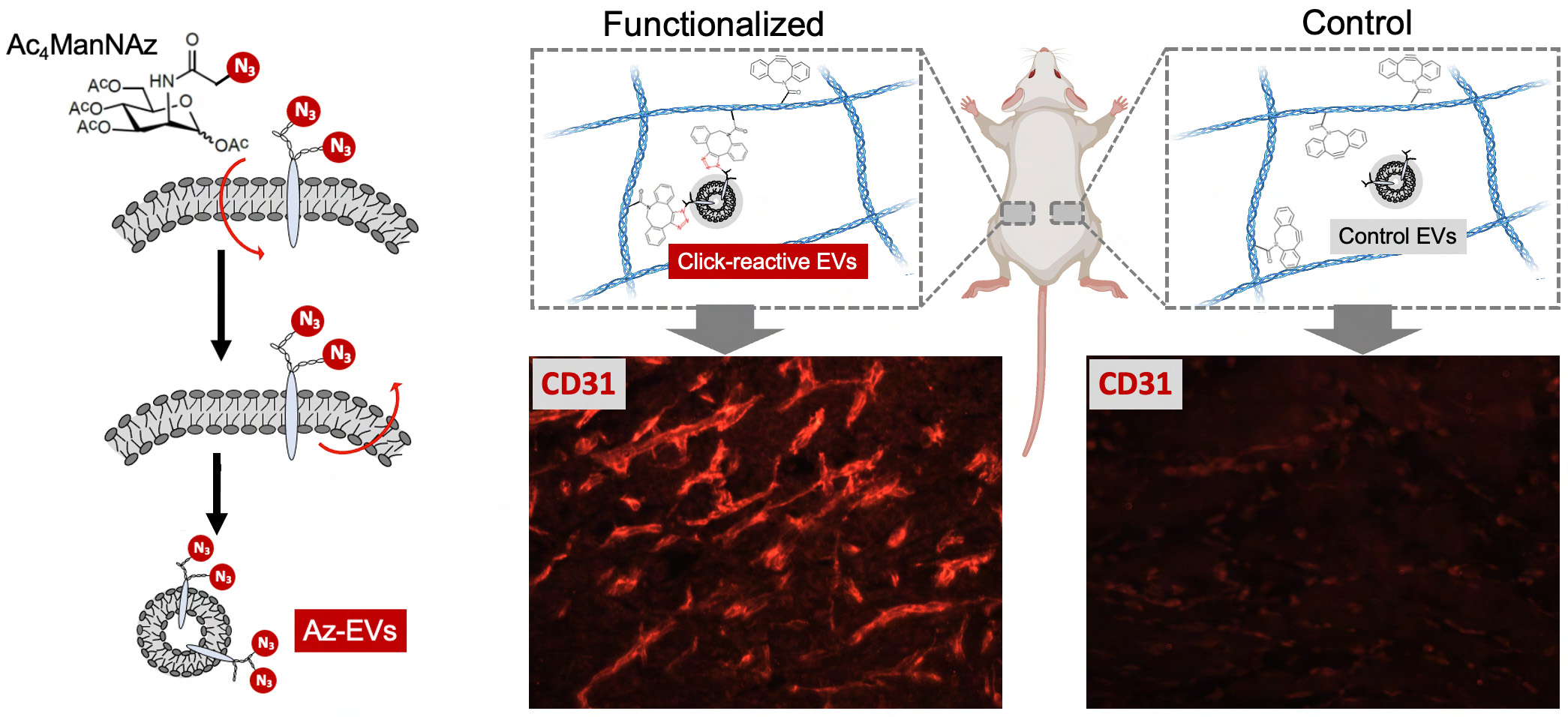
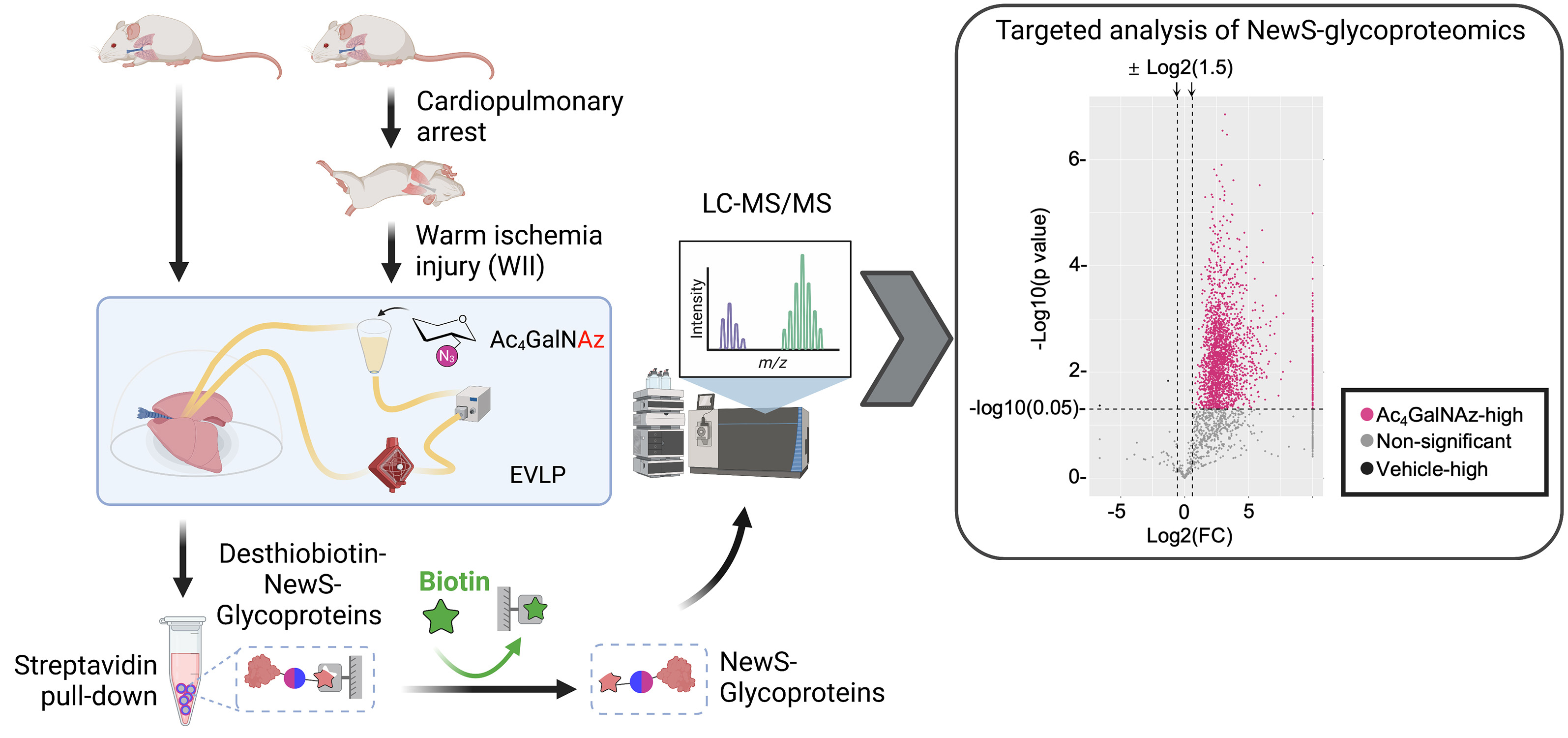
Engineering and Tracking the Extracellular Matrix (ECM). The ECM is a ‘nest’ built by cells for themselves to survive, function and communicate, and is the foundation of multicellular organisms. We have a long-term interest in deriving functional ECM materials that carry tissue-specific cues and in further functionalization of these materials in a chemoselective manner to endow them with novel functionalities without changing their bulk properties. To enable this effort, we have developed metabolic glycan engineering strategies for the native ECM (Ren X et al., 2018; Ling Z et al., 2021), extracellular vesicles (EVs) (Xing Y et al., 2022), and glycocalyx (Wijesekara P et al., 2021). In parallel, we have developed a complete proteomic pipeline for ultrasensitive tracking of ECM glycoprotein synthesis with the goal of capturing the dynamic changes of the extracellular microenvironment along organogenesis and pathogenesis (Ling Z et al., 2023). Ongoing research focuses on (1) spatially guided lung and vascular tissue morphogenesis using ECM-anchored guiding cues (such as EVs); and (2) revealing ECM and EV dynamics along lung development, aging, and tumorigenesis.